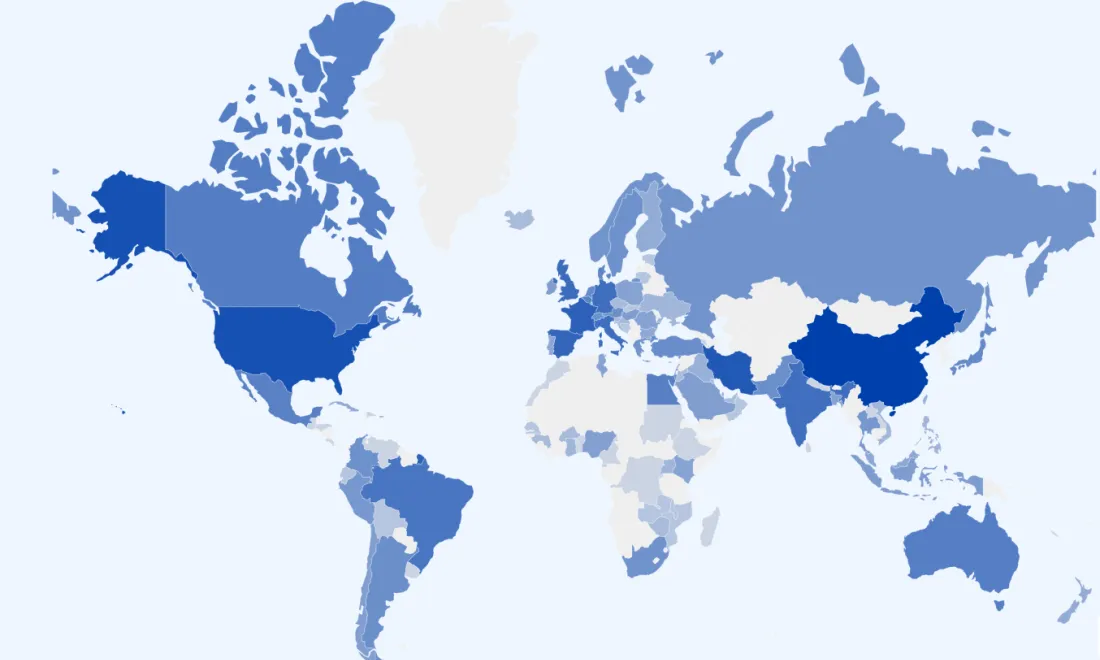
A mapping review of registered COVID-19 trials
Explore registered COVID-19 trials across the world in our interactive map. Use our Evidence Gap and Map (EGM) tool to identify ongoing trials, spot knowledge gaps, and avoid duplicating work.
We update the open access data in this map monthly, as part of a living mapping review in Wellcome Open Research.
The sheer quantity of ongoing research since the 2019 COVID-19 outbreak makes it difficult for researchers to evaluate and assimilate this work. Systematic reviews can help, but they provide a static snapshot in time, and can date quickly.
We instead use frequently updated living mapping review approach to summarise whole research landscapes, identifying knowledge gaps and informing future analyses. We update the COVID-19 EGM visualisation every month, constantly tracking therapeutic intervention studies.
Our COVID-19 EGM:
- Enables you to pull together and access information on COVID-19 trials across the world
- Includes detailed information on: study design, sample size, trial phase, active pharmaceutical ingredients (API) used, study location, and whether individual patient data (IPD) is available for reuse
- Is updated via every three months via a search and standardisation of the WHO International Clinical Trials Registry Platform (ICTRP).
What you can do with the EGM visualisation
Our EGM visualisation showcases the available metadata in three different ways: geography, lists and analytics which include descriptive statistical information. You can filter and search amongst studies to pinpoint work by the variables and studies that interest you. You can also compare studies within the EGM visualisation, and export data for further analysis.
As highlighted in our earlier analysis and its protocol, innovative EGM visualisations like these help prevent unnecessary duplication research.
Researchers have used this EGM visualisation to assess the distribution, design, size and other key outcomes of work COVID-19 therapeutic interventions such as ivermectin and hydroxychloroquine.
Acknowledgements
![]()
The COVID-19 EDM visualisation was supported by and developed in collaboration with the COVID-19 Clinical Research Coalition (CERCLE). IDDO is a founding member of CERCLE, which builds collaborative solutions and accelerates urgent research on prevention and diagnosis of COVID-19 in resource-limited settings. It provides free access to COVID-19 clinical trial protocols, and CERCLE working groups are identifying and addressing the most pressing scientific and operational research questions for resource-limited settings.