Contributing data
Many thanks for making your data available for reuse via IDDO/WWARN: this will help advance work in your field, as well as giving you an opportunity to participate high-impact collaborations and be credited in potential publications. Your institution/organisation continues to own the data you contribute. Find out more about reasons to share your data.
Read the IDDO Terms of Submission and make sure that you understand it. This is a legal document that sets out your rights and obligations as the data contributor, and do make sure that your institution/organisation (which will generally be the legal owner of data, the data controller) is onboard with the process too. You’ll be asked to tick a checkbox confirming you’ve read and agreed to these terms as you submit your institute’s data, and you will need to choose how you want requests to access the data handled: we encourage all data contributors to delegate this function to the independent IDDO Data Access Committee, but you can choose to review these requests yourself too.
Decide on a name for your dataset, and gather all files for it and any supporting information, including protocols, publications, data dictionaries, PubMed IDs (if your data is already published), plus any notes for our data managers.
Register for an IDDO account if you haven’t already got one. Registration is straightforward, and just needs your name, your organisation and an email you can access.
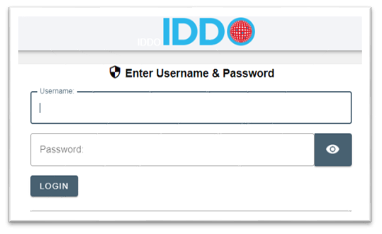
Login to our secure portal with the username and password that you created in the previous step:
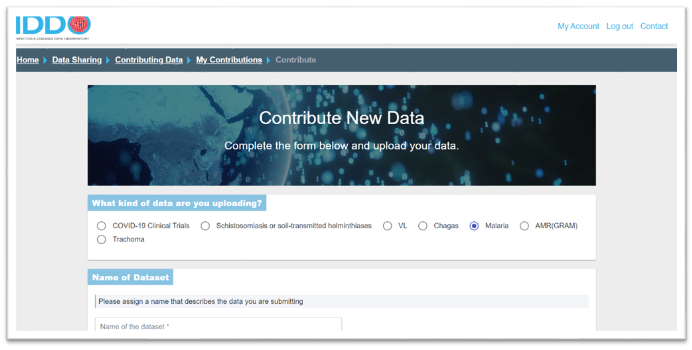
In the Contribute New Data section, choose the disease/theme to which you are contributing data (eg., malaria, AMR) by clicking on the appropriate radio button, and name your dataset (for example, AS/AQ vs AS/MQ study in Kampala, Uganda, 2014 or COVID-19 patient data, Central Hospital, Montreal, Canada, 2020 or MSF Kailahun ETU / PHE Labs Port Loko & Kerry Town)
You will then be directed to our Terms of Submission: choose one of the three signature options offered, indicating how you want requests to access your data handled. We encourage all data contributors to delegate this function to the independent IDDO Data Access Committee.
In the next section, include the name and contact information of the Data Contributors. This is important information: we will use these contact details for any queries about curation, to give you the option to get involved in any scientific collaborations using this data, data reuse requests and any other clarifications. You can also add further names as data controllers further along in the portal – see step 12.

Next, upload your data files in the Data Files section. CSV and Excel XLSX files are easier to handle and process, and we would suggest avoiding Word and PDF format data files if possible.
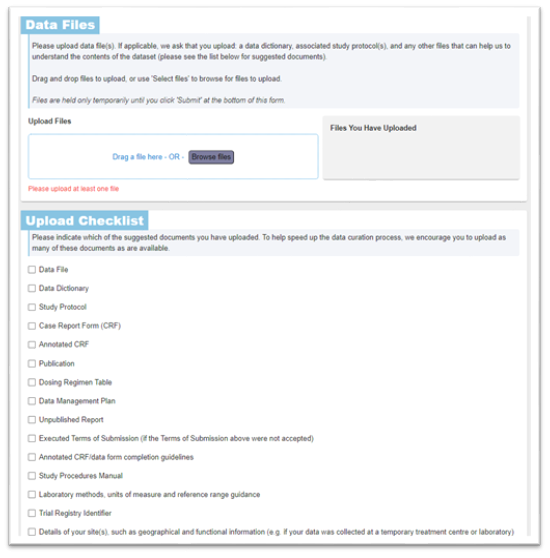
Include additional metadata about the individual patient data files in the ‘Upload Checklist’ section: you do not need to upload all of the documentation in this checklist, but do use this list as a prompt to consider what supporting information can optimise and facilitate data standardisation and make it easier for a researcher to reuse the data.
Include any trial registration in the ‘Do you have a Trial Registry Identifier(s)?’ section. This will also make it easier for researchers to find your data, increasing its reach. Its fine to include more one registry identifier. Also, upload any publications associated with the data – these can be draft manuscripts, journal publications, protocols, systematic reviews, etc.
Sharing data for a study group? Do include its name (it doesn’t have to be exact) under the ‘Sharing Data for a Specific Study Group or Project?’ section. This will help us link it to the right study group or project.
If your colleagues will be uploading further data files or responding to queries from us, include their email address under ‘Additional Administrator Access to this Dataset’. We may contact them if necessary, inviting them to create an account so they can see what files are uploaded, and add more if needed. You can provide as many additional contact email addresses as you’d like. Note that unlike Data Contributors, Data Administrators cannot make decisions about the data, but can support the data submission process. Eg. the PI of study as the Data Contributor can add a statistician/data manager from their group as an administrator.
Add any information that you think might help with our curation or governance of the data in the ‘Notes for IDDO Data Curators’ section.
You can also edit a dataset that you’ve already uploaded, going through all of these sections.
That’s it! Our data team will now curate your data into internationally recognised CDISC SDTM standards, to make reusing your data easier.
The time taken for this process depends on the complexity of your dataset: if your data needs to be available for reuse by a particular date, please get in touch with us well in advance, by emailing info@iddo.org.