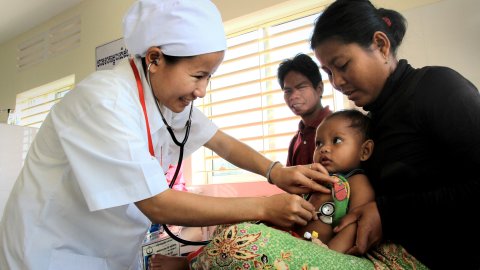
Study Groups
WWARN facilitates a number of collaborative Study Groups to undertake individual patient data meta-analyses to answer specific research questions about malaria treatments and antimalarial drug resistance. Gathering and combining data sets from multiple studies increases sample sizes, so that effects, including smaller effects, and effects on sub-populations can be identified with greater certainty. Working together and combining data from different regions and populations is improving our understanding of drug resistance and strengthening global efforts to control and eventually eliminate malaria.

i
Dominic Chavez World Bank

The study group aims to expand the evidence base on the safety of artemisinin-based therapies in the first trimester of pregnancy by consolidating and…
show more

The study group seeks to develop new methodologies for data integration of models for antimalarial drug resistance with disparate data sets.

We are undertaking an individual patient data (IPD) meta-analysis to compare the safety and tolerability of artesunate-pyronaridine vs artesunate-amod…
show more

This study group aims to define renal impairment in severe malaria in terms of expected mortality across a diverse patient population.

The aim of this study is to assess the effect of primaquine mg/kg dose and regimen duration on i) efficacy, ii) tolerability, and iii) safety in patie…
show more

The Study Group was formed in January 2024 to update and expand the WWARN work of the K13 Genotype-Phenotype Study Group, which was published in BMC M…
show more

This study group aims to determine the impact of malaria in the first trimester of pregnancy on the mother and infant through individual patient data…

Characterising the population pharmacokinetic properties of ivermectin in children and adults to inform optimal dosing of ivermectin
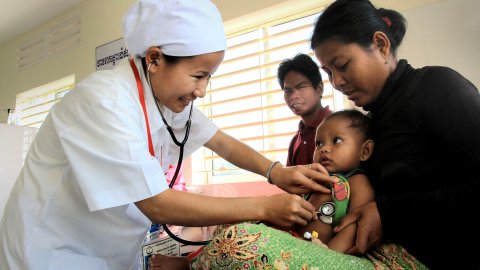
The paediatric single low-dose (SLD) primaquine safety study group aims to describe and compare the safety of SLD primaquine for transmission blocking…
show more

The paediatric single low-dose (SLD) primaquine efficacy study group aims to assess and compare the efficacy of SLD primaquine for transmission blocki…
show more

The Dihydroartemisinin-Piperaquine for Intermittent Preventive Treatment in Pregnancy Study Group aims to determine the safety and efficacy of intermi…
show more

Exploring the impact of malaria during pregnancy on infant anaemia, malaria, morbidity and growth – an individual participant meta-analysis.
Analysis of the effect of primaquine dose on tolerability and Plasmodium vivax recurrence.
The overall aim of the IPTp and SP-resistance-associated mutations Study Group is to quantify how parasites with the sextuple mutant pfdhps and pfdhfr…
show more
Assessment of relationship between Haemoglobin (Hb) and Haematocrit (Hct) measurements.

A meta-analysis of individual patient data (IPD) to determine the effect of antiretroviral (ARV) drug-drug interactions and HIV disease on artemether…

Determining the effects of pregnancy on piperaquine pharmacokinetics. The analysis aims to contribute evidence needed to inform recommendations on the…
show more

A pooled analysis of methaemoglobin as a marker of primaquine antihypnozoite activity in Plasmodium vivax malaria

The Plasmodium vivax fever study group aims to estimate parasitaemia thresholds for febrile patients who present for treatment and determine the…

Analysis of how age impacts the effect of primaquine dose on haematological safety in patients with Plasmodium vivax and Plasmodium ovale malaria
Analysis of how age impacts the effect of primaquine dose on gastrointestinal tolerability in patients with Plasmodium vivax and Plasmodium ovale mala…
show more

Analysis of how age impacts the effect of primaquine dose on efficacy in patients with Plasmodium vivax and Plasmodium ovale malaria

Analysis of the effect of primaquine dose on the efficacy, safety and tolerability in patients with Plasmodium vivax malaria
Determining what affects White Blood Cell (WBC) count at baseline and during acute phase of malaria infection.

Determining the optimal Artemether-lumefantrine antimalarial dosing for young children and pregnant women: A pharmacokinetic-pharmacodynamic meta…
show more

This Study Group will explore the link between P. vivax recurrence and prior P. falciparum treatment, including increased risk of vivax para…
show more

Assessing the efficacy of a range of antimalarials used for the treatment of P. falciparum malaria in all trimesters of pregnancy in Africa…
The First Trimester Safety of ACTs Study Group’s aim is to establish the evidence-base and safety profile to inform decision-making on the use of arte…
show more

Analysis of risk factors of Plasmodium vivax early and late recurrence. Published in July 2018.
Analysis of the consequences of symptomatic Plasmodium vivax infections on anaemia before and after antimalarial treatment

The Piperaquine Safety Study Group’s aim is to establish the evidence-base and safety profile to inform decision-making on the use and optimal dosing…

A pooled analysis on the relationship between K13 molecular marker and parasite clearance data. The Study Group closed to new participants in Dec…
show more
Pooled analyses of the efficacy and safety of single low-dose primaquine to interrupt P. falciparum malaria transmission.Data collection has closed an…
show more