CDISC Case Report Form development
As a key component of its data platform development process, IDDO works closely with research communities to develop a standard Case Report Form (CRF) tailored to each disease. The CRF does not prescribe what data to collect, but rather provides researchers with a standardised means of recording any data they do choose to collect from a study. This supports efficient, scientifically-valid generation and reporting of clinical data to streamline development of new treatments, regulatory submission and post-marketing research, as well as enabling data sharing, comparison and aggregation for high-quality, novel research outputs to address knowledge gaps.
Through the process described in the workflow below, IDDO works closely with researchers, regulators, pharma and policymakers with support from CDISC to develop consensus-based, freely-available data standards in compliance with existing CDISC standards.
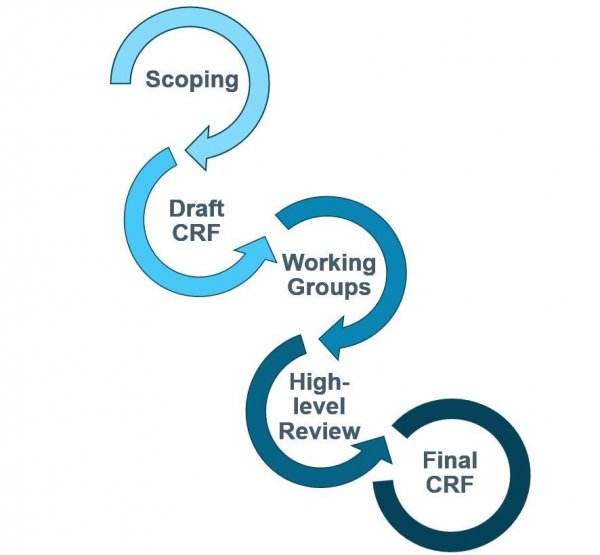
CRF Development Process
- Initial scoping of variables of interest for research theme
-
Collation of variables from extant CRFs
- Internal 1st draft informed by scoping
- Invite disease clinicians for review
- Finalise 2nd draft
-
Thematic working groups with disease clinicians
- Finalise 3rd draft
-
Invite multidisciplinary experts for review
-
Final review by key project partners and CDISC
- Finalise v1.0 CRF and User Guide
-
Published on the IDDO research theme and CDISC websites
-
Living document versioned for future iterations and expansion