Tools and resources
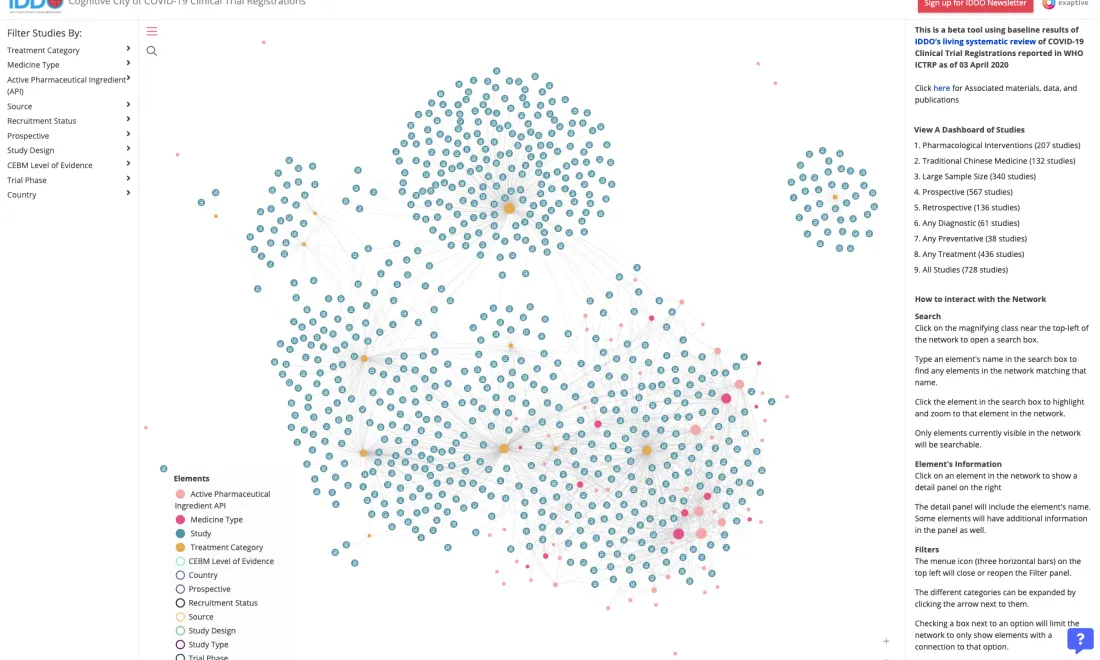
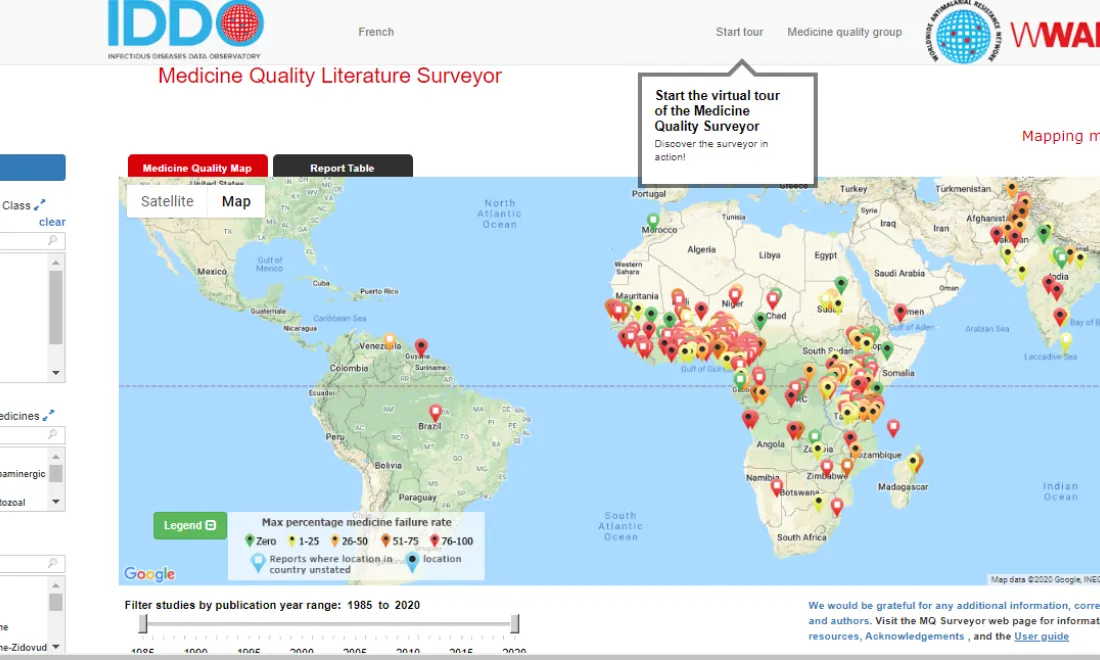
IDDO is committed to providing practical tools and services to support the collection of disease characterisation data and develop quality evidence on optimal treatment and response to emerging infections. Our resources help researchers gather and present evidence in a standardised way, and are intended to help facilitate a much faster, better coordinated and more effective response to future outbreaks. To find a wide selection of free online tools, services and resources that support malaria researchers to collect reliable and comprehensive evidence, visit the WWARN Toolkit.

i
Credit: WWARN