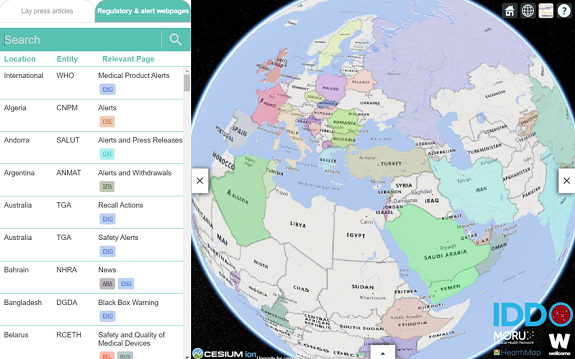
Regulatory and alert webpages
The ‘Regulatory & alert webpages’ section on the Medicine Quality Monitoring (MQM) Globe holds a list of webpages of regulatory authority or other institutions globally that contain relevant information on medical products quality issues, identified by the Medicine Quality Research Group.
The section on ‘Regulatory and alert webpages’ is a non-exhaustive list of Ministries of Health, Medicines Regulatory Authorities, and National Laboratories webpages that contain specific information on substandard and falsified (SF) medical products, currently covering 74 countries. The individual recalls/alerts listed on those webpages are not currently shown on the MQM Globe. The MQM Globe redirects to webpages with information on recalls, safety alerts on SF medicines, illegal products, product quality problems in general, warning letters to manufacturers, etc.
Regulatory authorities are sharing information in different ways on their websites. Currently only ‘dedicated webpages’ are accessible through the MQM Globe. We hope to include the ‘non-dedicated webpages’ soon. The section with ‘Regulatory & alert webpages’ is subject to ongoing improvement.
- Dedicated webpages
Currently the ‘Regulatory & alert webpages’ section only contains URL-links directing to dedicated webpages on medical product quality issues. Please note that due to their structure, some webpages the Globe directs to, do not directly display the list of recalls/alerts. The user thus needs to navigate through the webpage to find the desired information. -
Non dedicated webpages
Some regulatory authorities publish their SF alerts on a news webpage or a general alert or a general news section of the Ministry of Health or even the general government webpages. Currently webpages that are not exclusive to medical product quality issues are not included on the ‘Regulatory & alert webpages’ of the Globe
Please take into account the following caveats when navigating through the data in the ‘Regulatory & alert webpages’ section:
- Not all regulatory authorities/governments have webpages. The MQRG may have not identified some existing webpages. Thus some countries do not currently have webpages available on the ‘Regulatory Webpages’ section.
- For certain countries and/or entities, we only display certain types of information (for example batch recalls) because we did not find publicly available information on other types of incident (for example seizures of falsified medicines).
- Some authorities only supervise certain product categories (e.g. medicines but not necessarily medical devices) or they do supervision of several but the webpage identified by our team only contains information for one category.
- The website of a regulatory authority does not necessarily contains the same information in different languages. It might be that in ‘language A’ a dedicated webpage is available but that in ‘language B’ only a non-dedicated webpage is available. Nevertheless the link to ‘language B’ is displayed on the Globe.
Any remarks on the design or additions to content of the Globe are greatly appreciated, please write to medicinequality@iddo.org