Visceral leishmaniasis
Visceral leishmaniasis (VL) is a neglected tropical disease that threatens tens of thousands of people each year, mostly in the world’s poorest communities. Left untreated, it is fatal in over 95% of cases.
At IDDO, we work with the global VL research community to pool and harmonise clinical trial data, creating stronger evidence to improve treatments and support new drug development. Together, we also develop the tools, resources and research agendas that drive better VL research and save lives.

Contributing data
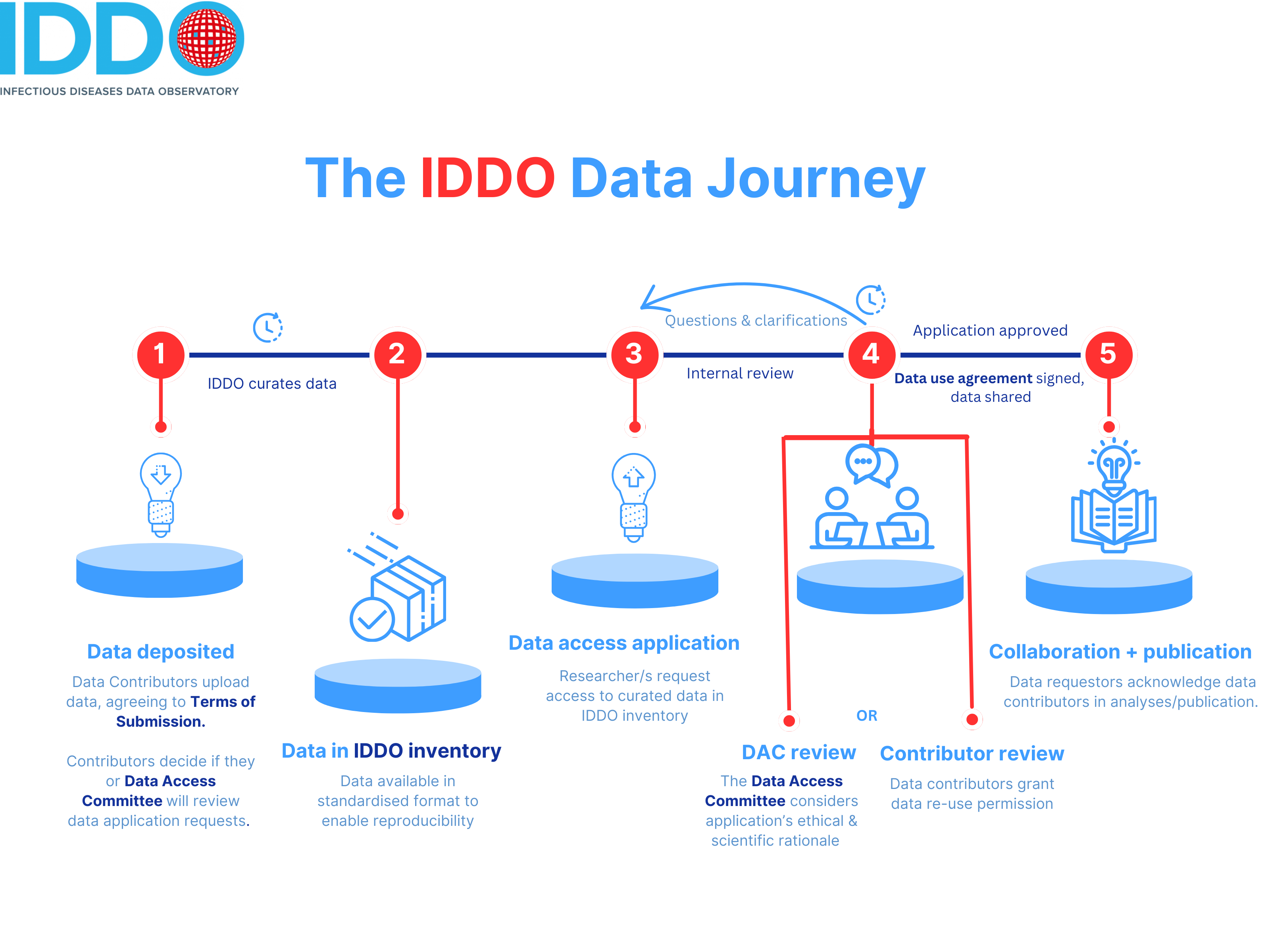
Many thanks for making your data available for reuse via IDDO/WWARN: this will help advance work in your field, as well as giving you an opportunity to participate high-impact collaborations and be credited in potential publications. Your institution/organisation continues to own the data you contribute. Find out more about reasons to share your data.
Read the IDDO Terms of Submission and make sure that you understand it. This is a legal document that sets out your rights and obligations as the data contributor, and do make sure that your institution/organisation (which will generally be the legal owner of data, the data controller) is onboard with the process too. You’ll be asked to tick a checkbox confirming you’ve read and agreed to these terms as you submit your institute’s data, and you will need to choose how you want requests to access the data handled: we encourage all data contributors to delegate this function to the independent IDDO Data Access Committee, but you can choose to review these requests yourself too.
Decide on a name for your dataset, and gather all files for it and any supporting information, including protocols, publications, data dictionaries, PubMed IDs (if your data is already published), plus any notes for our data managers.
Register for an IDDO account if you haven’t already got one. Registration is straightforward, and just needs your name, your organisation and an email you can access.
Login to our secure portal with the username and password that you created in the previous step:
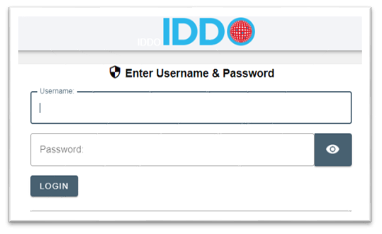
In the Contribute New Data section, choose the disease/theme to which you are contributing data (eg., malaria, AMR) by clicking on the appropriate radio button, and name your dataset (for example, AS/AQ vs AS/MQ study in Kampala, Uganda, 2014 or COVID-19 patient data, Central Hospital, Montreal, Canada, 2020 or MSF Kailahun ETU / PHE Labs Port Loko & Kerry Town)
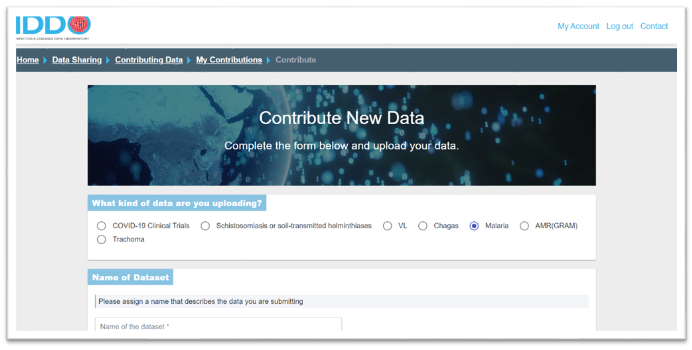
You will then be directed to our Terms of Submission: choose one of the three signature options offered, indicating how you want requests to access your data handled. We encourage all data contributors to delegate this function to the independent IDDO Data Access Committee.
In the next section, include the name and contact information of the Data Contributors. This is important information: we will use these contact details for any queries about curation, to give you the option to get involved in any scientific collaborations using this data, data reuse requests and any other clarifications. You can also add further names as data controllers further along in the portal – see step 12.

Next, upload your data files in the Data Files section. CSV and Excel XLSX files are easier to handle and process, and we would suggest avoiding Word and PDF format data files if possible.
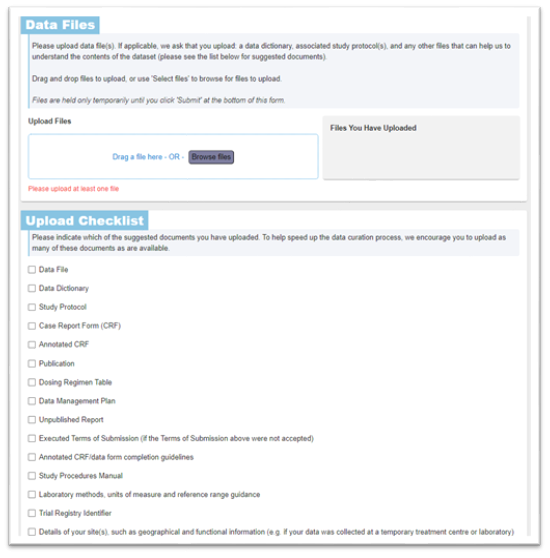
Include additional metadata about the individual patient data files in the ‘Upload Checklist’ section: you do not need to upload all of the documentation in this checklist, but do use this list as a prompt to consider what supporting information can optimise and facilitate data standardisation and make it easier for a researcher to reuse the data.
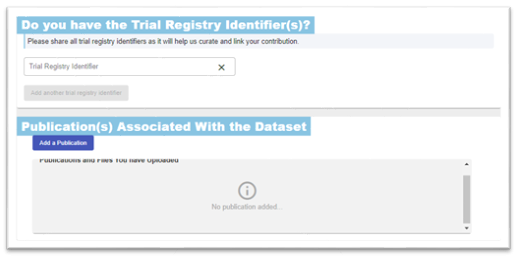
Include any trial registration in the ‘Do you have a Trial Registry Identifier(s)?’ section. This will also make it easier for researchers to find your data, increasing its reach. Its fine to include more one registry identifier. Also, upload any publications associated with the data – these can be draft manuscripts, journal publications, protocols, systematic reviews, etc.
Sharing data for a study group? Do include its name (it doesn’t have to be exact) under the ‘Sharing Data for a Specific Study Group or Project?’ section. This will help us link it to the right study group or project.
If your colleagues will be uploading further data files or responding to queries from us, include their email address under ‘Additional Administrator Access to this Dataset’. We may contact them if necessary, inviting them to create an account so they can see what files are uploaded, and add more if needed. You can provide as many additional contact email addresses as you’d like. Note that unlike Data Contributors, Data Administrators cannot make decisions about the data, but can support the data submission process. Eg. the PI of study as the Data Contributor can add a statistician/data manager from their group as an administrator.
Add any information that you think might help with our curation or governance of the data in the ‘Notes for IDDO Data Curators’ section.
You can also edit a dataset that you’ve already uploaded, going through all of these sections.
That’s it! Our data team will now curate your data into internationally recognised CDISC SDTM standards, to make reusing your data easier.
The time taken for this process depends on the complexity of your dataset: if your data needs to be available for reuse by a particular date, please get in touch with us well in advance, by emailing info@iddo.org.
Accessing data
IDDO’s data repositories include thousands of datasets across many diseases, which are available, for free, to any researcher across the world to use in this their work.
Accessing data is straightforward: complete a short form outlining the data and variables you need, and your planned scientific use. Each request is reviewed by an independent expert committee, with the aim of facilitating responsible data access.
Start by browsing the available data in your disease or research area of interest:
- WWARN / Malaria data
- Visceral leishmaniasis (VL) data
- COVID-19 data
- Chagas disease data
- Antimicrobial resistance (AMR) data
- Schistosomiasis / soil-transmitted helminths (Schisto/STH) data
- Ebola data
Make a note of the IDDO study IDs and variables you wish to request. You’ll need these in the application.
Your application will be reviewed either by the independent IDDO Data Access Committee or by the original data contributors, depending on the dataset.
The committee includes subject matter experts across all disease areas and is guided by a commitment to facilitating responsible data access.
We recommend reviewing the data access assessment criteria as set out in the Data Access Guidelines before you apply.
Think through the key details of your project. You’ll need to provide:
- A clear research question and scientific rationale
- Names and roles of your research team
- Methodology, analysis plan, and outcome measures
- Ethical approvals or guidelines that apply
- Your publication and dissemination plans
This information will be submitted along with your selected study IDs and variables in the Data Access Application form.
Download and fill in the Data Access Application Form, then email it to dataaccess@iddo.org. We’ll review your form, request any additional information if needed, and then share it with the relevant review body.
Note that when you submit a data access application or contribute data to IDDO’s data repositories, IDDO may use the information provided to invite you to participate in surveys designed to enhance our services. Participation in such surveys is voluntary, and no personal data is collected through these activities.
Before we can share data, you or an authorised signatory at your institution will need to sign a legally binding Data Use Agreement (DUA).
Please read this carefully and ensure your team complies with the terms.
Cite IDDO and the relevant dataset DOIs in all outputs. Let us know if you’re presenting or publishing work using IDDO data—we’re always happy to help amplify your research.
Email info@iddo.org or tag us on BlueSky or LinkedIn.
Start with our FAQs, or email dataaccess@iddo.org with any questions.
Tools & resources
Explore a collection of tools and resources to support harmonised data collection, better data management, data analysis and more robust research for VL. Email info@iddo.org for more information.


We bring together VL researchers worldwide in collaborative study groups to identify the most important questions for patient benefit. We then build on this with our expertise in curating and standardising data, which enables individual participant data meta-analyses

The IDDO Visceral Leishmaniasis theme is governed through the IDDO Board, an expert Scientific Advisory Committee, the independent IDDO Data Access Committee, and the IDDO senior management and team leaders. Together, they provide strategic oversight, independent scientific guidance, objective decisions on data access, and day-to-day management of the platform.
Design, Conduct, Analysis, and Reporting of Therapeutic Efficacy Studies in Visceral Leishmaniasis: A Systematic Review of Published Reports, 2000–2021
A systematic review (SR) of published efficacy studies in visceral leishmaniasis (VL) was undertaken to describe methodological aspects of design, conduct, analysis, and reporting. Studies published during 2000–2021 and indexed in the Infectious Diseases Data Observatory VL library of clinical studies were eligible for inclusion (N = 89 studies). Of the 89 studies, 40 (44.9%) were randomized, 33 (37.1%) were single-armed, 14 (15.7%) were nonrandomized multiarmed studies, and randomization status was unclear in two (2.2%). After initial screening, disease confirmation was done by microscopy in 26 (29.2%) and by a combination of serology and microscopy in 63 (70.8%). Post-treatment follow-up duration was <6 months in three (3.3%) studies, 6 months in 75 (84.3%), and >6 months in 11 (12.4%) studies. Confirmation of relapse was solely based on clinical suspicion in four (4.5%) studies, parasitological demonstration in 64 (71.9%), using molecular/serological/parasitological method in 6 (6.7%), and there was no information in 15 (16.9%). Of the 40 randomized studies, sample size calculation was reported in only 22 (55.0%) studies. This review highlights substantial variations in definitions adopted for disease diagnosis and therapeutic outcomes suggesting a need for a harmonized trials protocol.
Blood transfusion in the care of patients with visceral leishmaniasis: a review of practices in therapeutic efficacy studies
Abstract
Blood transfusion remains an important aspect of patient management in visceral leishmaniasis (VL). However, transfusion triggers considered are poorly understood. This review summarises the transfusion practices adopted in VL efficacy studies using the Infectious Diseases Data Observatory VL clinical trials library. Of the 160 studies (1980–2021) indexed in the IDDO VL library, description of blood transfusion was presented in 16 (10.0%) (n=3459 patients) studies. Transfusion was initiated solely based on haemoglobin (Hb) measurement in nine studies, combining Hb measurement with an additional condition (epistaxis/poor health/clinical instability) in three studies and the criteria was not mentioned in four studies. The Hb threshold range for triggering transfusion was 3–8 g/dL. The number of patients receiving transfusion was explicitly reported in 10 studies (2421 patients enrolled, 217 underwent transfusion). The median proportion of patients who received transfusion in a study was 8.0% (Interquartile range: 4.7% to 47.2%; range: 0–100%; n=10 studies). Of the 217 patients requiring transfusion, 58 occurred before VL treatment initiation, 46 during the treatment/follow-up phase and the time was not mentioned in 113. This review describes the variation in clinical practice and is an important initial step in policy/guideline development, where both the patient's Hb concentration and clinical status must be considered.