i
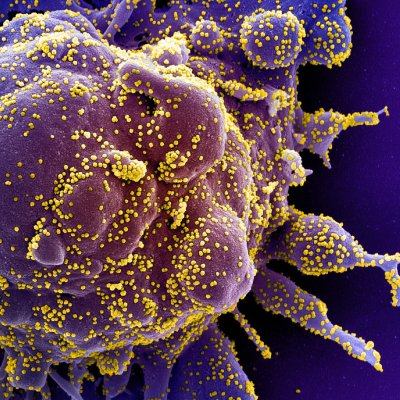
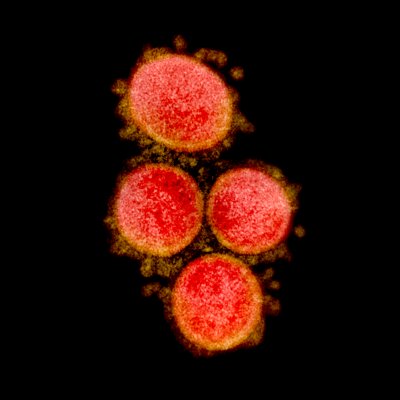
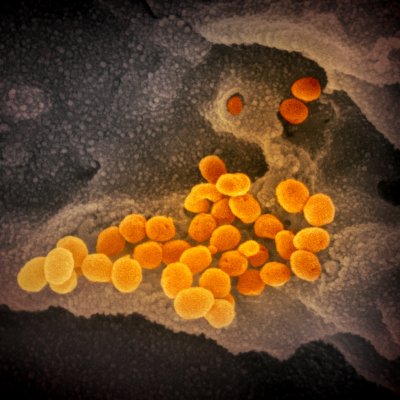
Credit NIH
We host one of the largest international collections of clinical data related to COVID-19 with detailed individual patient data on more than 800,000 hospitalised individuals from over 1,200 institutions across over 60 countries. IDDO has facilitated the re-use of these data in 60 novel analyses to date.
Read more about our COVID-19 partners
Explore the COVID-19 Clinical Research Coalition, a global research response to COVID-19 driven by the needs of low-resource settings.
Explore our COVID-19 publications
News