Tracking Resistance to Artemisinin Collaboration (TRAC I)
Tracking Resistance to Artemisinin Collaboration (TRAC I)
The project spent three years investigating the spread of parasite resistance to artemisinin-based therapies. Coordinated by the Mahidol Oxford Tropical Medicine Research Unit (MORU).
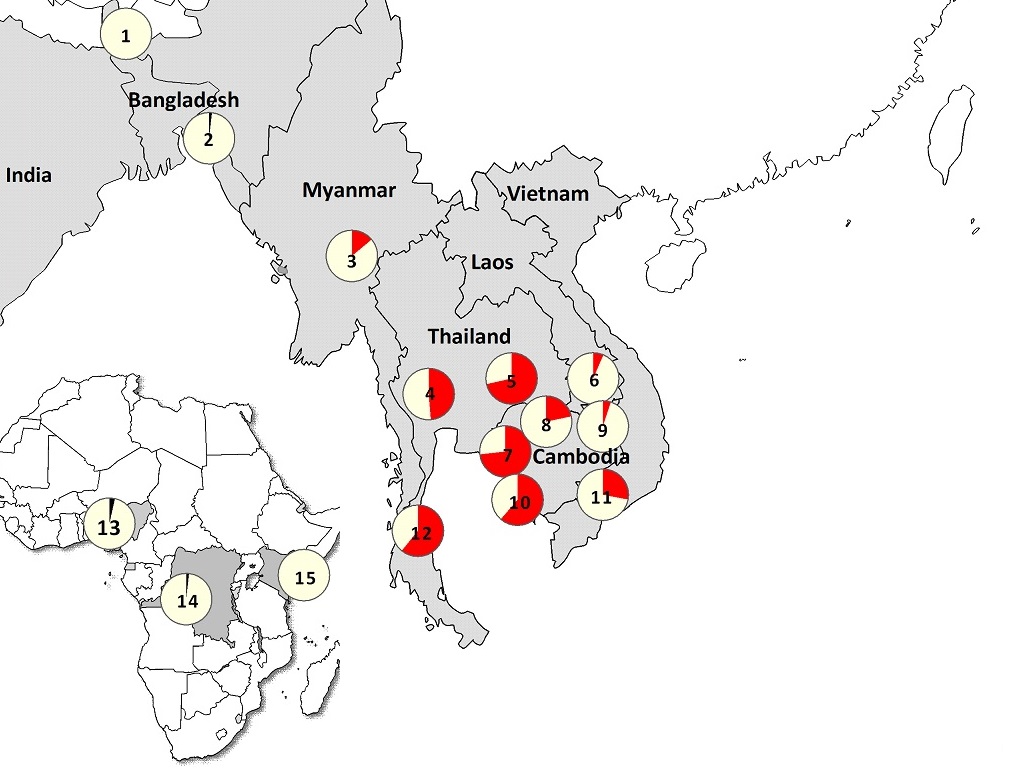
Researchers studied clinical, molecular, pharmacokinetic and socio-economic aspects of artemisinin resistance at 15 sites, mainly across Asia but also at three locations in Africa. The study confirmed that artemisinin resistant parasites are now firmly established in Western Cambodia, Thailand, Vietnam, Eastern Myanmar and Northern Cambodia. There are also signs of emerging resistance in Central Myanmar, Southern Laos and Northeastern Cambodia.
Concurrent research on vector control and demand side issues will fill knowledge gaps and contribute to the planning of the next steps in the response to artemisinin resistance.
The three-year TRAC project was funded by a grant from the UK Government Department for International Development (DFID). Partners include: Mahidol Oxford Tropical Medicine Research Programme (MORU); London School of Hygiene and Tropical Medicine (LSHTM); Liverpool School of Tropical Medicine (LSTM); WHO Global Malaria Programme; and the Worldwide Antimalarial Resistance Network (WWARN). Professor Nicholas J White is Principal Investigator and the project is co-ordinated by Dr Elizabeth Ashley.
WWARN researchers were responsible for quality assurance such as specimen collection, processing and tracking, and microscopy, as well as contributing to the overall operational and scientific coordination of the TRAC study. A WWARN-managed Specimen Management Centre at Mahidol University in Bangkok archives clinical samples from surveillance studies and clinical trial sites, including TRAC.
The project had four arms:
1. Clinical research
Data and samples collected from the study sites determined whether artemisinin resistance had spread outside Thailand and Cambodia, by identifying sites where slow parasite clearance is observed.
Patients with acute falciparum malaria were randomised to either 3 days of oral artesunate 2mg/kg or 4mg/kg daily. Parasitaemia was measured at six-hourly intervals until negative. Artesunate was followed by the standard ACT course used at the site. Blood samples were also drawn for parasite DNA and RNA storage, parasite cryopreservation and measurement of plasma artesunate and dihydroartemisinin concentrations.
Several complementary approaches were used to identify molecular markers and resistance mechanisms including candidate marker genotyping, genome-wide association studies, signatures of selection studies, transcriptional profiling and metabolite analysis. Parasites were cryopreserved to create a repository so that other researchers may study any identified resistance phenotypes, work to identify biomarkers of resistance that can be used as tools for surveillance, including molecular markers, and to try to identify mechanisms of resistance.
As a key partner, WWARN was responsible for the following activities coordinated by WWARN Molecular and the Asia Regional Centre, and in close collaboration with the Mahidol-Oxford Research Unit, Bangkok, Thailand.
- Management of the Specimen Management Centre
- Quality assurance and training in laboratory and clinical procedures
- Developing procedures for collecting and preserving samples for molecular analysis, including parasite DNA and RNA: training in their use
- Study site assessments (see TRAC Training Videos box)
2. Demand Factors
Led by LSHTM, epidemiological studies assessed demand factors at selected clinical sites, including surveys of antimalarial drug quality and availability.
- Illness episodes and treatment seeking behaviour were studied in Cambodia. An epidemiological and behavioural survey collected and mapped permanent household locations and areas where patients are most likely to have been infected. This provided information on patients’ risk factors for acquiring malaria, including main areas of transmission, treatment seeking behaviour, and will be complementary to the clinical trial findings.
- In collaboration with ACTWatch - Cambodia, samples of antimalarials and “drug cocktails” have been collected in a nationwide drug outlet survey. Drug quality and availability, including artemisinin monotherapies, will be assessed at all other TRAC clinical sites
- To understand demand in the context of antimalarial drug use and resistance, an economic and policy literature review informs a framework for analysis that will identify areas for policy intervention.
- A multi-level map of malaria indices, health systems and population movement in Cambodia is being constructed in partnership with the Partners for Development (PFD), the National Centre for Parasitology, Entomology and Malaria Control, Cambodia (CNM), Population Services International (PSI), Clinton Health Access Initiative (CHAI), WHO and other partners.
3. Vector Control
- Focused on Cambodia, surveys of LLIN usage were complemented by an entomological survey in Pramoy, Pursat Province where artemisinin resistance is prevalent. Lead by LSTM, the objective is to assess the malaria vector species abundance, local distribution and seasonal variation over a complete year. Different sampling methods will be evaluated. The study will provide data on infectivity and insecticide resistance of mosquito species.
- Studies of novel vector control interventions, run in parallel to the entomological surveys, will inform larger scale trials of one or more of these interventions including a device offering mosquito protection in a defined space, a novel repellent space spray using agricultural technology used successfully to clear susceptible crop pests from large orchards, and insecticide treated clothing and hammocks.
4. WHO and TRAC
WHO Global Malaria Programme has provided the study artesunate from a quality assured batch, and also provided ACTs for sites in Laos and Myanmar.
Use our kelch markers toolkit which recommends minimal criteria for reporting Plasmodium falciparum kelch13 (pfkelch13) markers for reporting artemisinin drug resistance.
Elizabeth A Ashley et al. Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. Advanced online publication in New England Journal of Medicine. July 31 2014; 371:411-23. DOI: 10.1056/NEJMoa1314981.